Osmolality is a valuable measurement tool. Together with our partner, Advanced Instruments, we share our knowledge about it to refresh your knowledge or even gain new insights.
What is osmolality?
Simply put, osmolality is a measurement of the total number of solutes in a liquid solution expressed in osmoles of solute particles per kilogram of solvent. You can test the osmolality of many solutions, including serum, urine, plasma and biologics. You use a special device called an osmometer to test osmolality.
Freezing Point depression osmometers are the industry-preferred solution in clinical chemistry, pharmaceutical and quality control labs. Read more and learn all about it.

Osmolality in Biopharma
Used as an in-process control and quality-check throughout development and manufacture of biologics.

Osmolality in Clinical
To test osmolality of serum, plasma and urine because it provides information on the body’s hydration state, used to invegate low sodium levels, and to detect the presence of toxins in the body.
I&L Biosystems knows osmolality
Meet I&L Biosystems
Osmolality is a valuable measurement tool. Together with our partner, Advanced Instruments, we share our knowledge about it to refresh your knowledge or even gain new insights. I&L Biosystems offers competent advice, application specific solutions and comprehensive service for your laboratory. Our key focus is in the areas of Microbiology, Cell Biology, Biotechnology and Process Control.
Our partner in osmolality:
Advanced Instruments
With Advanced Instruments, we know you’ll have the best in class osmometry equipment. It has over 60 years of experience and is an early pioneer in the field of osmometry. During that time they have become a trusted valuable reference in the science of osmolality testing.

How it’s used
Biopharmaceutical labs use osmolality testing as an in-process control and quality-check throughout development and manufacture of biologics. Osmolality testing helps ensure cell health, in-process reagent quality and helps reduce the risk of batch failures.
About osmolality in Biopharma
To ensure a quality manufacturing process is achieved, the use of process analytical technology (PAT) is recommended. Osmolality as a PAT can be used to monitor raw material components, process intermediates and finished products. Osmolality is a measure of solute concentration in process fluids and is considered a crucial quality attribute and critical value in bioprocessing.
Read all about this Freezing Point technology in Biopharma in the white paper Osmolality in Biopharma and application note Osmolality as a quality attribute in downstream processing. Both compiled by Advanced Instruments.
Preserving data integrity: 21 CFR Part 11 compliance and osmolality as a process parameter
A collaboration of Advanced Instruments with Angela Bazigos, CEO of Touchstone Technologies Inc.
What you will learn:
-
Explanation what data integrity is for pharma and biotech industry.
-
Demonstrate how your analytical instruments can be 21 CFR part 11 and EU Annex 11 compliant for data integrity and electronic record management.
-
Recognize the value of osmolality testing as a key process parameter in pharma and biotech industry and how it supports 21 CFR part 11 and EU annex 11 regulations.
8 ways osmolality testing improves cell and gene therapy
Cell and gene therapy has come a long way in a short amount of time and there is a desire to identify ways to consistently improve manufacturing workflows. Osmolality offers not just one, but eight ways to build confidence in your bioprocess.

Or check out the webinar!
What you will learn:
-
What role does osmolality play in viral vector stability?
-
How is osmolality testing used in viral vector UF/DF?
-
What are some use cases for osmolality in cell culture monitoring & QC?
-
How does osmolality keep cells stable during cryopreservation?
Reliable osmolality testing of high concentration mAb formulations
The Jefferson Institute of Bioprocessing (JIB) evaluated Advanced Instruments’ newest freezing point depression osmometer, OsmoTECH® XT, against vapor pressure technology to analyze mAb formulations & salt standards. Curious about the test results? Download the application note.

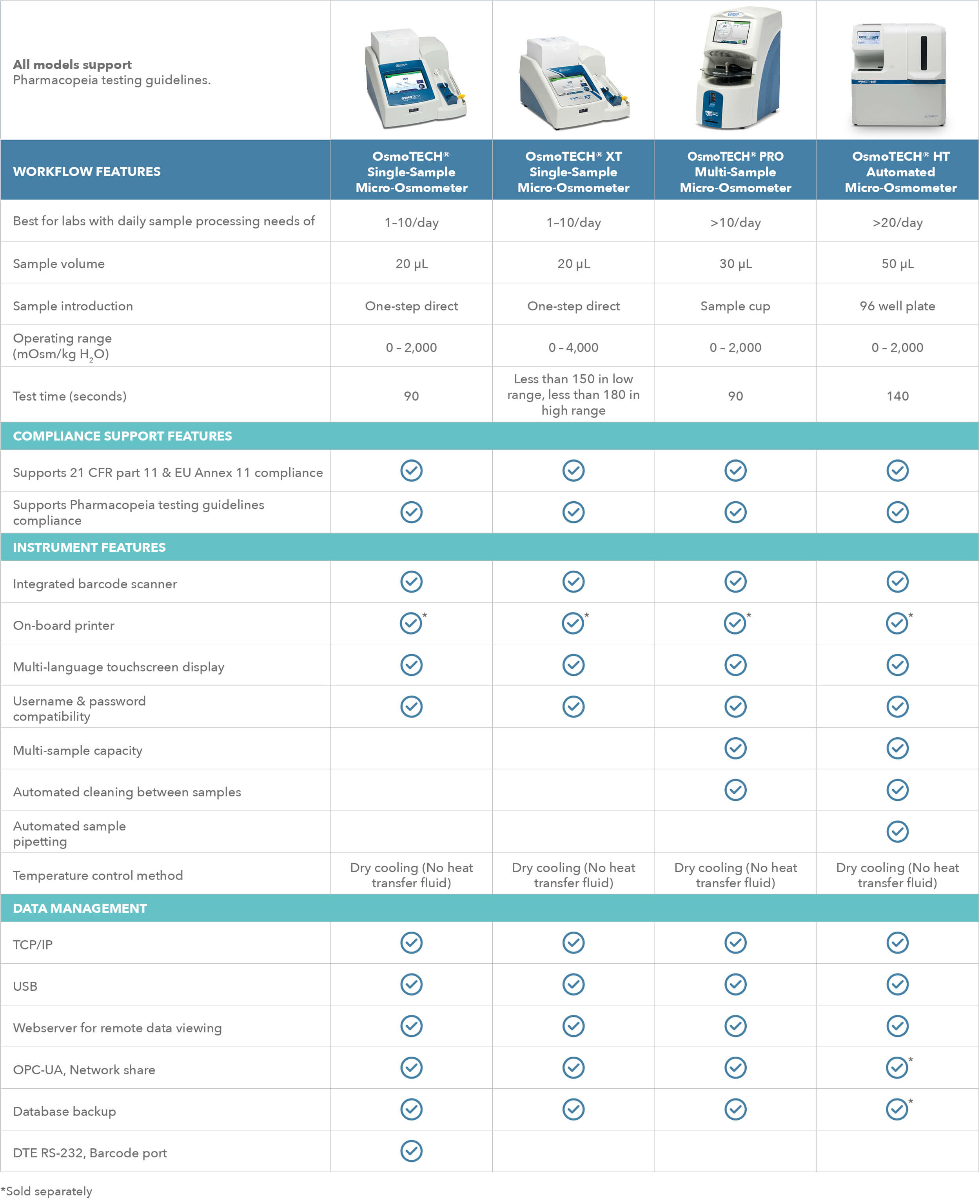
All Advanced Instruments osmometers for Biopharma
From knowledge to knowhow, I&L Biosystems shares an overview of all osmometers from our partner in osmolality: Advanced Instruments. State of the art osmometry equipment meets the latest requirements. They are an early pioneer in the field with over 60 years of experience and are always ahead of the market.
Need some help or a good advice? Want a demo? Click here and we’ll get in touch.
How it’s used
Clinical labs test osmolality of serum, plasma and urine because it provides information on the body’s hydration state to detect the presence of toxins and to monitor patients receiving osmotically active drug therapies. It is a valuable clinical tool in the diagnosis and treatment of patients.
About osmolality in Clinical
Osmolality is a fundamental measurement of the total solute concentration of body fluids, including but not limited to serum, plasma, urine, whole blood and stool. Measuring osmolality is critical when you suspect toxin ingestion, electrolyte disorders and metabolic acidosis. Osmolality testing as an initial screening can help reduce cost and improve quality of care*.
Read all about Freezing Point technology in Clinical in this white paper compiled by Advanced Instruments.
*The Value of osmolality Testing; Neville R. Dossabhoy, MD, FACP, FASN, Consultant Nephrologist
Osmolality & COVID-19: The valuable role of osmolality testing in COVID-19
A Scientific Resource from Advanced Instruments
Osmolality testing can help with early diagnosis and etiological determination of hyponatremia, which is critical for this patient population.
Summary article
Recent publications have shown that COVID-19 (coronavirus disease 2019), an infectious disease caused by a novel coronavirus known as severe acute respiratory syndrome coronavirus (SARS-CoV-2), is associated with electrolyte disorders including hyponatremia. Early diagnosis and etiologic determination are critical in any hyponatremic patient to ensure proper treatment and to avoid potential harm to the patient.
Osmolality testing is quick, inexpensive and effective to help with early detection and diagnosis of hyponatremia. Osmolality testing can help ensure that appropriate treatment strategies are implemented early for hyponatremic patients which may be even more significant in patients hospitalized with COVID-19. As clinicians and researchers continue to expand their understanding of COVID-19, additional laboratory testing is important to consider to help better understand and diagnose a COVID-19 patient’s underlying conditions.
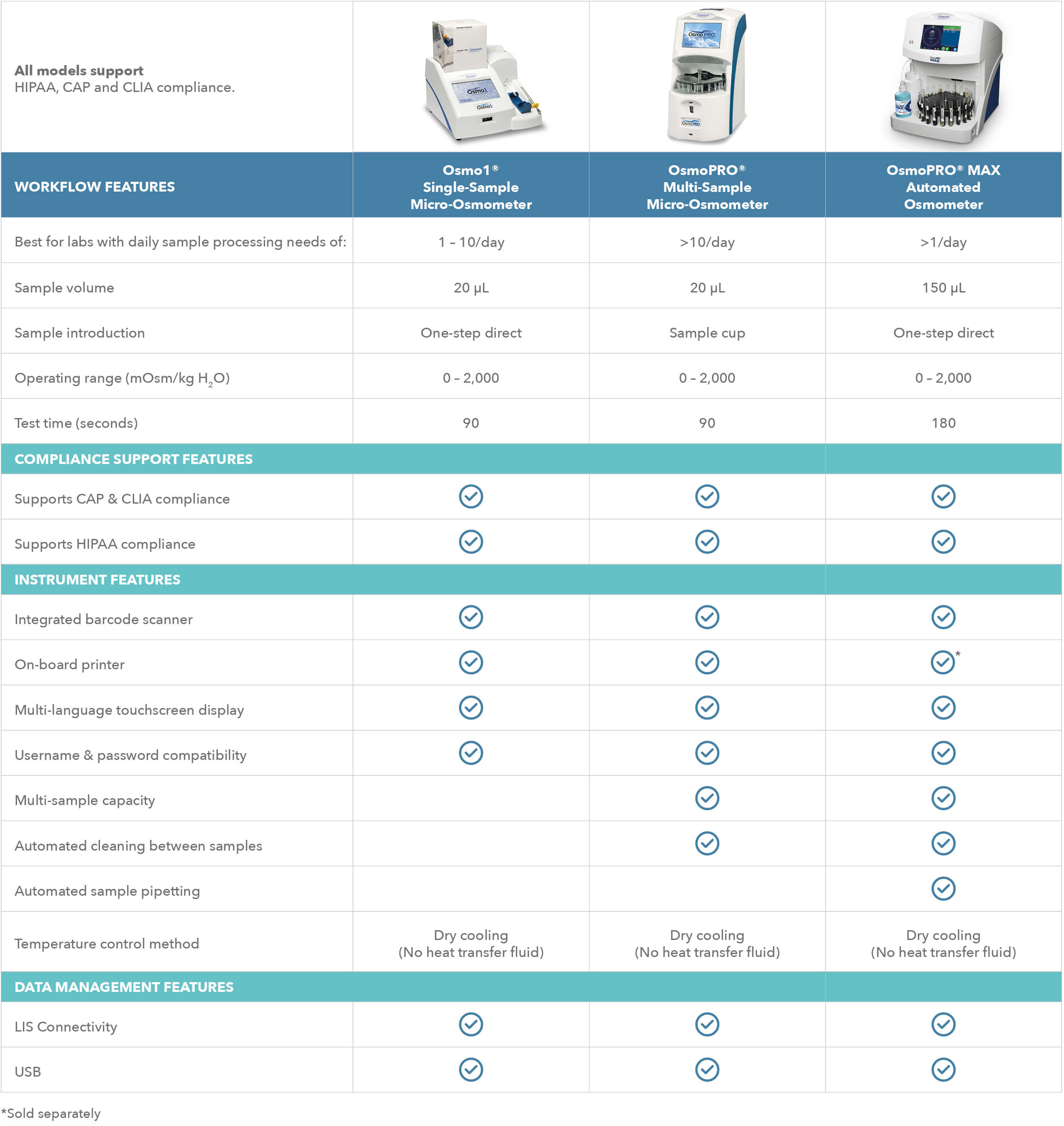
All Advanced Instruments osmometers for clinical
From knowledge to knowhow, I&L Biosystems shares an overview of all osmometers from our partner in osmolality: Advanced Instruments. State of the art osmometry equipment meets the latest requirements. They are an early pioneer in the field with over 60 years of experience and are always ahead of the market. Need some help or a good advice? Want a demo? Click here and we’ll get in touch.

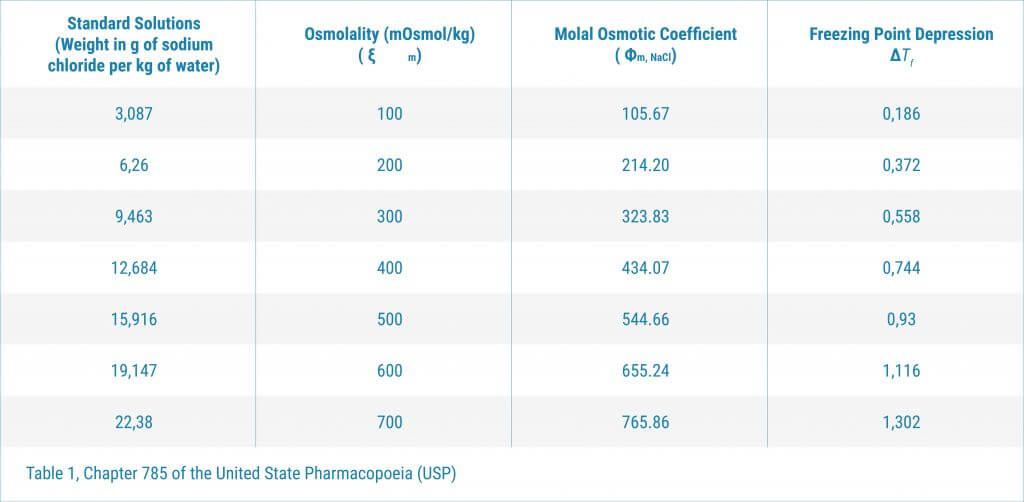
 Osmolality is a great tool in biopharmaceutical development. However the rules of using the equipment differ in the EU from the US. Key differences:
Osmolality is a great tool in biopharmaceutical development. However the rules of using the equipment differ in the EU from the US. Key differences: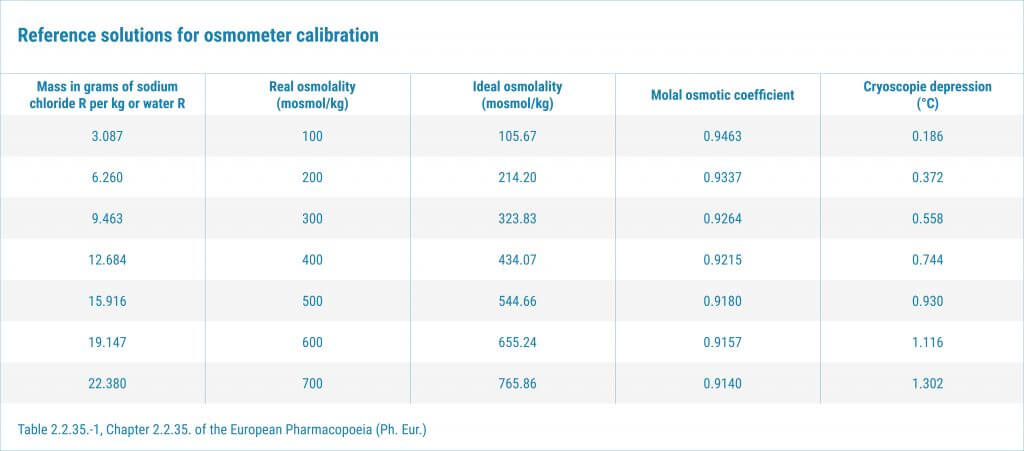
 Do you recognize this? Your research is going well. The results are looking good and you are about to finalize your valuable work. But then you discover that your osmometer is dated and it does not comply with the adapted regulations. It even causes your workflow to become non-compliant. Your workflow reached its Freezing Point.
Do you recognize this? Your research is going well. The results are looking good and you are about to finalize your valuable work. But then you discover that your osmometer is dated and it does not comply with the adapted regulations. It even causes your workflow to become non-compliant. Your workflow reached its Freezing Point.