Downstream processing remains one of the most time consuming and labour intensive step in pharmaceutical manufacturing. With a special focus chromatography, on separating and processing the API, protein or fragment, this poses a promising and significant chance for time and cost cuts, efficiency increases and process streamlining. Radial flow chromatography can achieve much more with less.
Contents of this page
What is radial flow chromatography?
To find out how the radial flow chromatography technology works and how precisely it can benefit most current downstream purification applications, we have to find out how it actually works and how this working mode of operation differs from traditional or axial column technology.
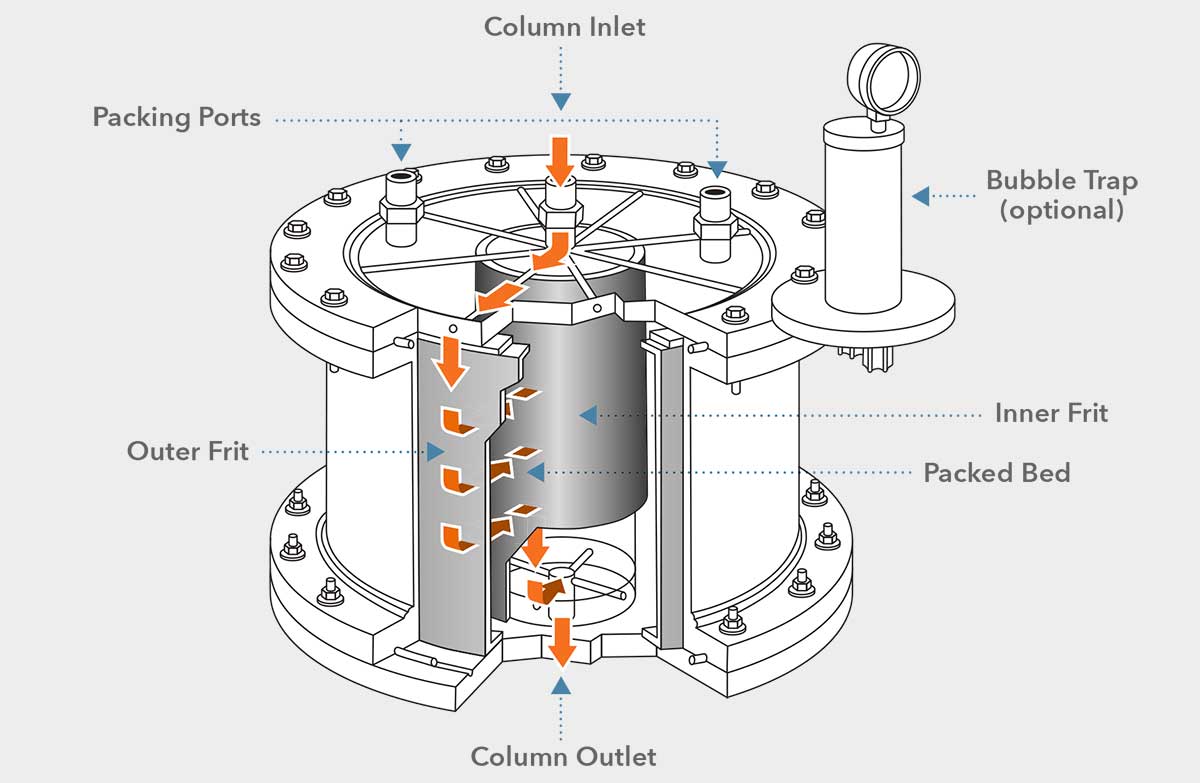
The first and very apparent significant difference to the widely known axial flow columns is the flow distribution across the bed and column. While in axial chromatography it can be as simple as so called 'gravity flow', meaning the flow being led top to bottom of the column with the force of gravity as a driving force and as sophisticated as to having to have intricate distribution technology at the top head of the axial column to ensure an even distribution of the sample upon entering the short but wide column bed (in preparative /industrial chromatography), the sample moves across the bed horizontically with radial flow technology. The eluent / sample enters at the top of the column, the top lid acts as a distribution head and directs the sample into the gap between the outer column body and the outer frit.
It distributes over the whole height of the column and flows horizontal from the outer frit to the inner core. From the gap between the inner frit and the collector rod (spacer in the center of the column) the eluent leaves through the outlet at the bottom of the column. The effective bed height of the column is the distance between inner and outer frit. This results, of course, in a fixed bed height, unlike plunger utilizing systems often found on axial columns. While having to have a certain amount of chromatographic resin (typically 120% of the nominal column volume) ready for any sort of separation experiment with a radial flow column, it is, by the fixed bed length and fixed volume (there also isn't a plunger to seal the top of the column), any separation experiment stays highly reproducible. Additionally, once validated, the volume and all conditions for sepraration on the respective column stay constant without room for error without ever having to readjust the column bed.
The principle of radial flow chromatography was developed and patented by Sepragen as early as 1985. Sepragen's SuperFlo radial flow chromatography columns are characterised by their high efficiency and the unique, extremely fast and uncomplicated packing process (See "How are radial flow columns packed?"). In addition, the processes are easily scalable to production scale (See "How does upscaling for production work?"). Sepragen's SuperFlo radial flow chromatography columns are made of acrylic or stainless steel and are offered in sizes from 50 ml to 1500 ml for laboratory scale and from 5 L to 500 L for production.
What is the difference between radial flow chromatography columns and axial columns?
The driving force of this type of chromatography is the pressure difference between the inner (smaller) and the outer (larger) frit, converging with their corresponding difference in surface area, resulting i a differential velocity. With the flow across a respective surface area increasing towards the center of the column, meaning the later /inner end of the bed exhibits a higher backpressure than the earlier /outer parts of the bed, leading solutes across the bed horizontally in a precise manner. With surface areas significantly increasing compared to axial columns, this column design allows for higher flow rates with similar linear velocities, keeping the interaction time between solid phase and eluent intact.
Another important point to mention as an inherit difference in chromatographic performance between axial and radial flow chromatography is the distribution of eluent flow. While (at least larger scale) axial columns need their eluent to rely on often complicated flow distribution across the inlet head before entering the column bed through the first frit, the position as to where the eluent molecule enters the chromatographic bed is irrelevant and results in precisely the same condition /separation time and distribution as any other eluent molecule. With no further potentially failing /clogging distribution technology, the eluent distribution across the axial flow column bed results in a widened gaussian curve, since taking the fastest and shortest way across inlet head, column bed, outlet head can significantly differ from taking the widest and slowest way (outer ends of the column). This results in lower separation resolution and difficulties detecting potential column bed shortcomings. With the radial flow, the eluent always travels horizontally across the column bed and since the sum of the way from inlet head to entering the column bed and way from exiting column bed to the outlet head is always constant, there is a higher resolution to be found inherit in this column design.
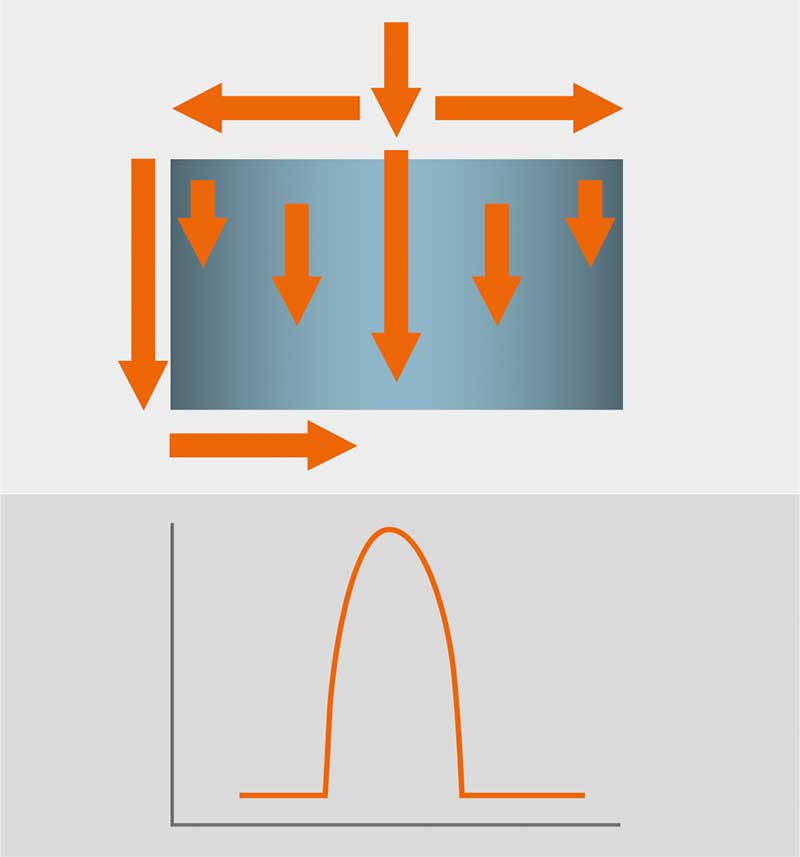
Figure 2: Result – the wider the column the broader the peak.
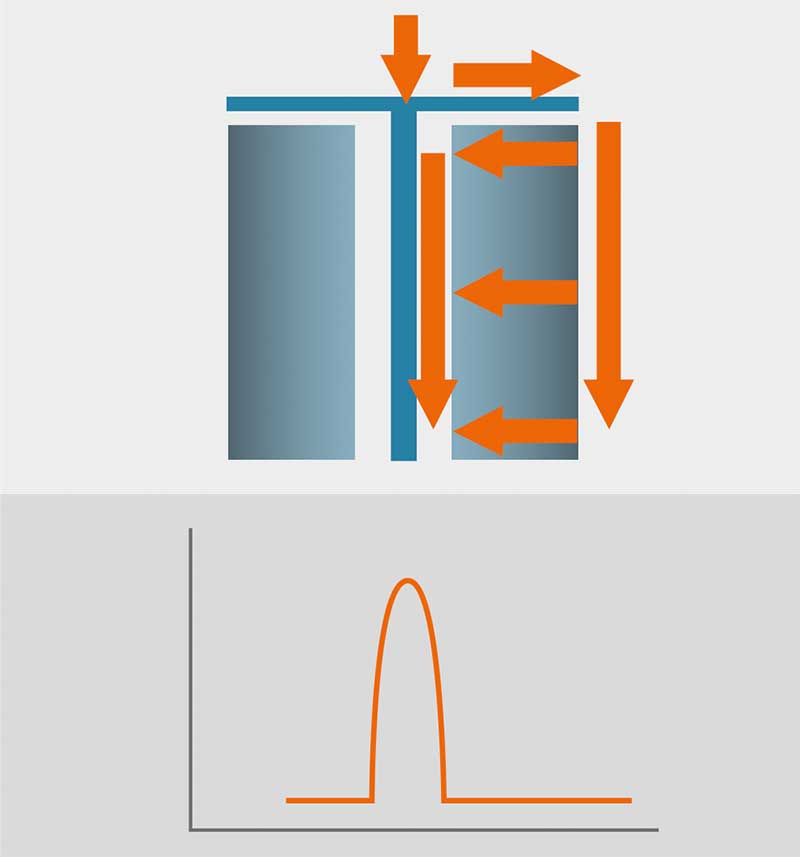
Figure 3: Result – Peak width is consistent
How to translate a process from axial flow chromatography
Typically, when transferring processes or upscaling a process with a change in bed height, which would be not uncommen in axial flow chromatography processes, the separation factor that anchors the transfer to a constant is the residence time, or the time an eluent has for interaction with the stationary phase (or rather a typically normal distribution of it). A separation similar to a small scale experiment can still take place as long as the interaction between eluent and stationary phase is long, high, often enough. Since the bed height is defined per engineering and cannot be altered, the remaining parameter to estimate and target a set residence time is the beforementioned linear velocity. Since this critical parameter does not remain constant across the column bed with radial flow technology, the extremes can be taken as the flow across the surface of the inner (highest linear velocity per volume = lowest residence time) or outer frit (lowest linear velocity) area. Most commonly and most practically, the most desirable flow rate /linear velocity can be estimated as a ring within the column bed in between the two frits and maintained and, if desired, scaled up upon keeping the bed height and column technology the same. With a nonlinear distribution of the linear velocity, radial flow technology ensures highest process fidelity and flexibility with its inherit design and phyiscal attributes.
How does Radial flow chromatography speed up my process?
As briefly outlined as a characteric in column design, the larger surface area with a commonly short bed (as often found in preparative chromatography) results in a more even distribution of the eluent, a much lower backpressure at the same linear velocity and therefore, a potential to speed up separation of the same size significantly. This effect is only enhanced when there are process steps that do not need to be limited by a certain linear velocity, such as equilibration of the column with the mobile phase and potentially wash steps in between load and elution of the sample of interest.
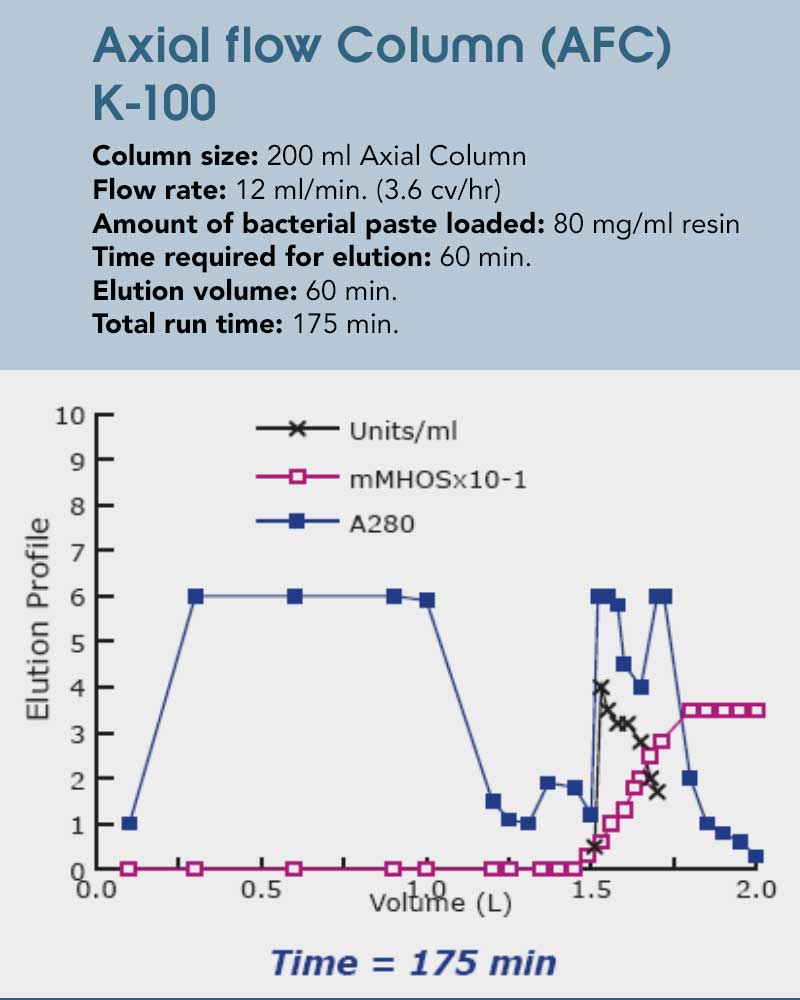
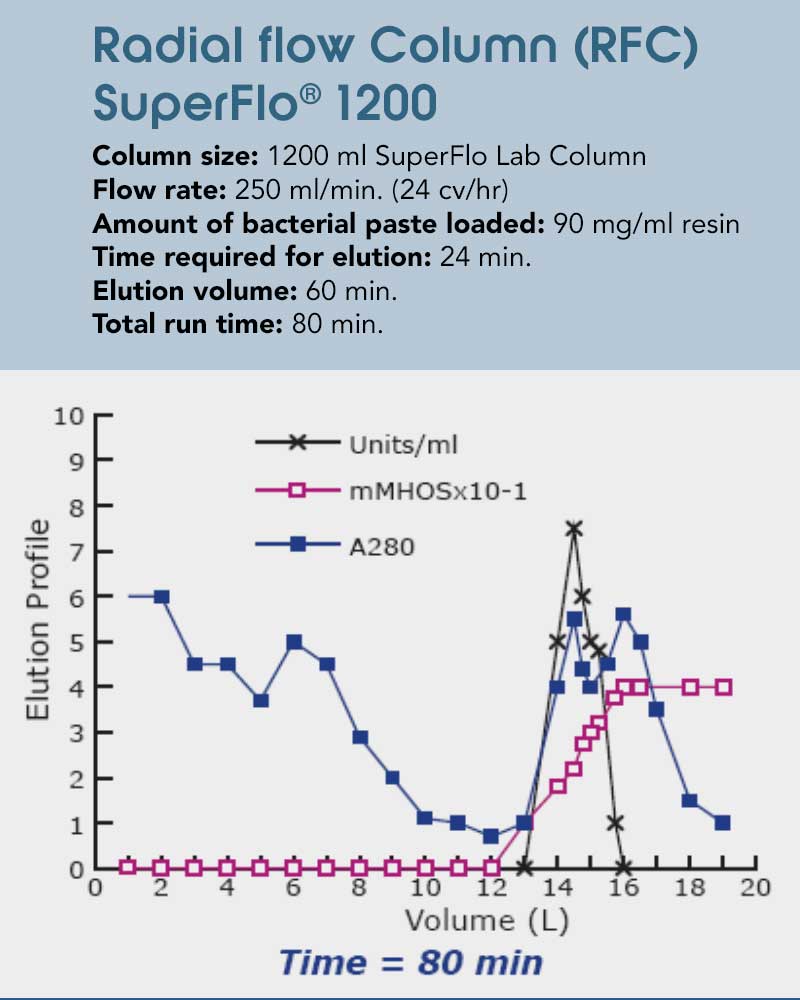
The following case study explores the potential for time savings utilizing the unique radial flow characteristics on the purification of an intracellular bacterial enzyme. While the column used here exhibits a much higher volume that the axial flow one, the bed height remains constant which results in similar separation parameters. With the drastically larger surface area and low backpressure, with the radial flow column the entire separation process can be finished in less than half of the time. That is taking a constant elution flow (as per column volume) and the advantages of a faster flow while washing and equilibrating into account. The maximum flow rate here is almost 7x as high, demonstrating the significant capabilities utilizing radial flow in any sort of chromatography process involving a chemical interaction between mobile and stationary phase can have. Additionally, not only is the processing time decreased, but the recovery is enhanced due to reduction of on column proteolytic degradation, resulting in 10% more loading capabilities.
Figures 4+5: The example above shows the isolation of a recombinant protein. Not only is the processing time decreased, but the recovery is enhanced due to reduction of on column proteolytic degradation.
How can radial flow chromatography save resin?
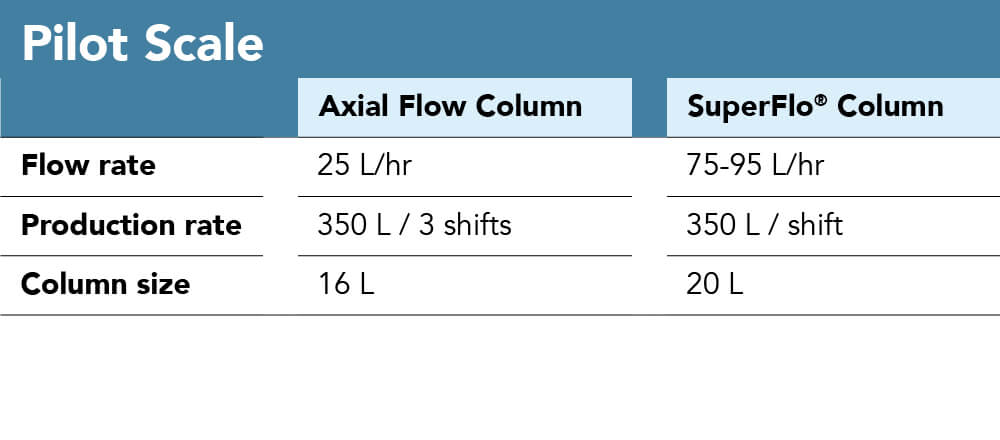
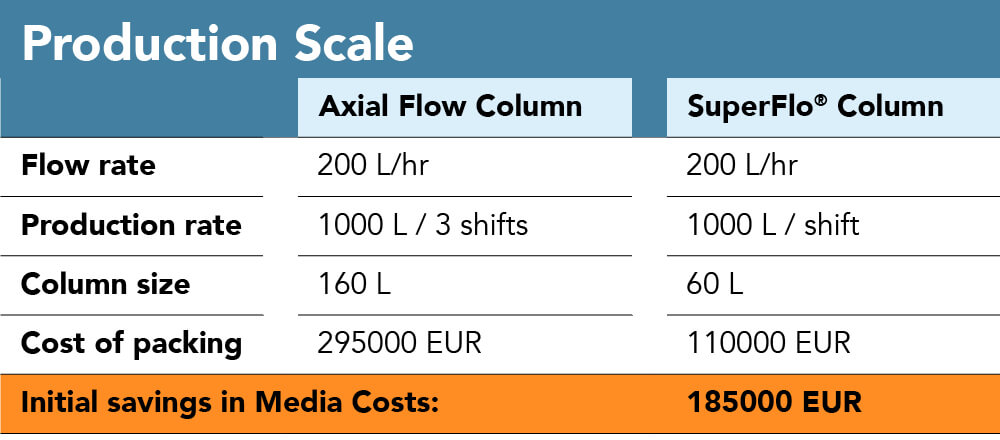
We established the inherent advantage the radial flow design has in terms of processing times and limitations in terms of pressure or linear velocity. This can result in much higher throughput at the same time and with the respective similar column size, if an increase in product amount is desired but there physical parameters can additionally be exploited to keep a similar throughput at a reduced material investment. This is to say with a decrease in column size, keeping the yield constant, there is potential so reduce resin amount, labour hours, column footprint. As a numerical example, the 200 L/h purification could either be done ona 160 L axial flow column in a 3-shift rotation or a 60 L radial flow column within a single shift.
As another use case and one of the most common applications in radial flow chromatography established with accomplished pharmaceutical companies, there is room to reduce cost of the process with blood plasma fractionation. The data below was obtained from a plasma fractionation facility. The study was performed in order to compare the performance of a 20 L radial flow column with that of a 16L axial flow column. With utilization of radial flow chromatography, flow rates increased over 3-fold with a corresponding increase in separation speed from three shifts to one shift without affecting product recovery or purity. Costs here are presented to be an estimate, however, decreasing column size (volume) by more than half of the initial (and returning) investment, potential savings are significant.
Figure 6: Process parameters for both pilot and production scale axial vs. radial flow chromatography with the objective of scaling down production vessel and process sizewhile maintain yield and throughput to withhold investments for resin. While in the pilot scale, with a similar column volume, the yield could be drastically increased, yield is maintained at the production scale comparison to highlight column size difference (160 L vs 60 L) and the corresponding potential resin savings.
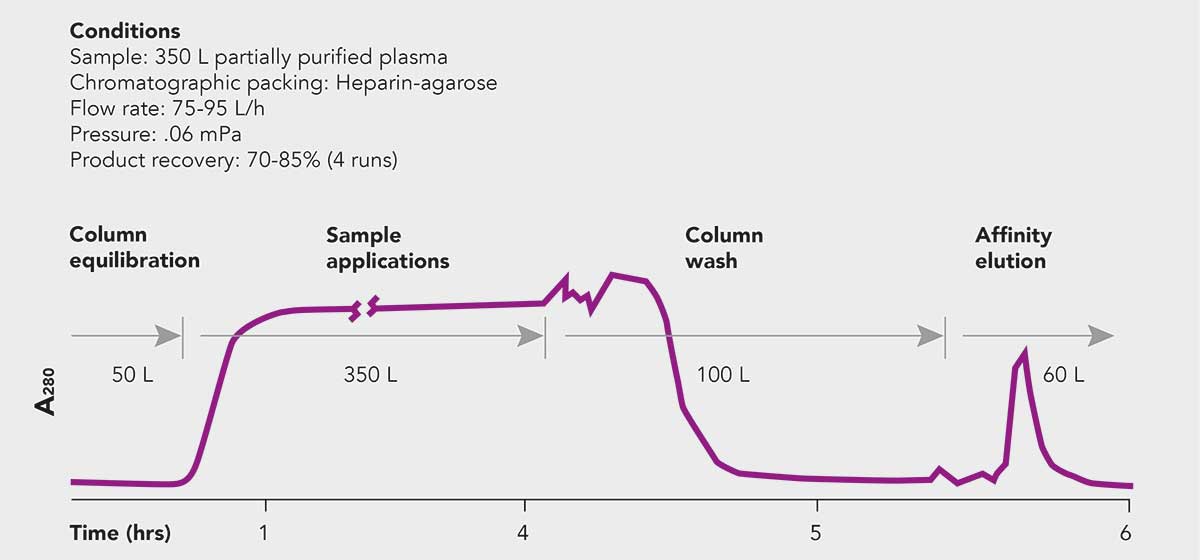
Figure 7: Elution of 350 L of partially purified plasma run on a SuperFlo® 20 L column packed with heparin-agarose. The data was obtained from a plasma fractionation facility. The study was performed in order to compare the performance of a Superflo® 20 L Column with that of a 16 L axial flow column. Table 1 shows a comparison of the performance of the two columns. With the Superflo® Column, flow rates increased over 3 fold with a corresponding increase in separation speed from three shifts to one shift without affecting product recovery or purity. (Courtesy: Bayer Corp.)
How does upscaling for production work?
A significant development factor in downstream processing is upscaling. Once established on a research scale, protocols need to be adjusted, tested and validated on a larger scale to ramp up production for the appropriate process and harvest rate. As column sizes grow, most of the time the bed height changes with it, making it difficult to translate the research process to the industrial scale. As frit surface area (per volume) changes, the linear velocity needs to be reevaluated. As the bed length changes, backpressures, flow rate and separation accuracy needs to be closely monitored and even adjusted.
An important invention to remedy those potential inaccuracies with radial flow chromatography can be the so called wedge column. As the name suggests, it represents a cut out part of an entire radial column, however, keeping the bed height and the frit surface are ration from inner to outer frit constant and comparable to a process scale complete radial flow column. That way, the volume is reduced by the factor of the total height of the column and the factor of the wedge angle compared to the 360° design of a complete radial flow column. Essentially, this means all separation parameters stay congruent with the upscaling process as long as the flow rate is scaled up by the same factor the volume to the final larger scale column is calculated with. In the case study, the same concentration of bovine serum was loaded onto differently sized columns. One column is a 400 mL wedge column, the other a 12 L complete radial flow column, resulting in the upscaling factor of 30. Just by multiplying the sample size and the flow rate by this factor, the wedge column gives a superimposable chromatogram to the one obtained in the final test with the actual column.
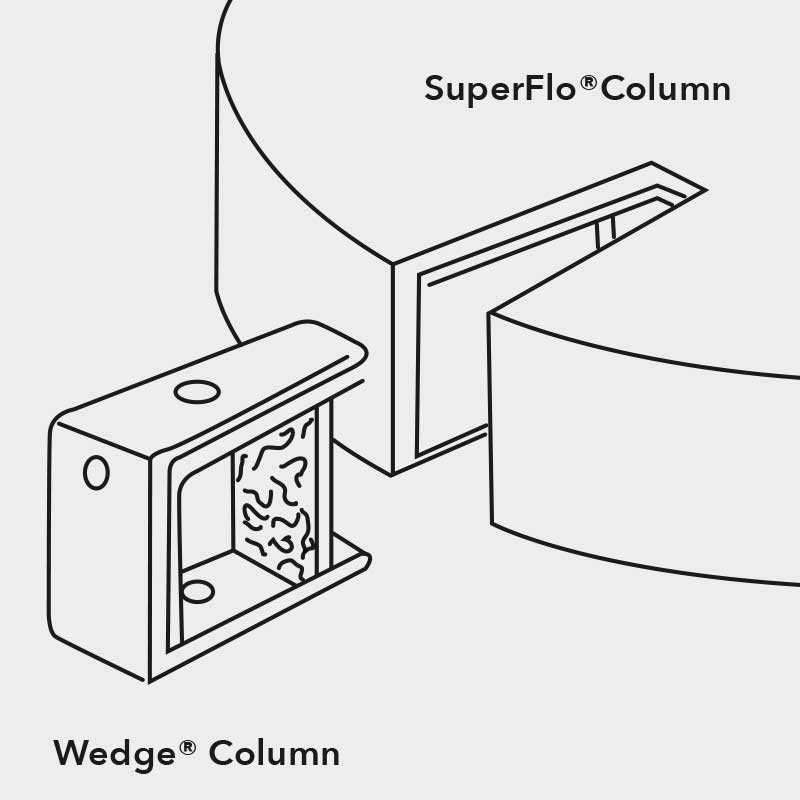
Figure 8: The Wedge column represents a cut-out piece of a radial flow column the desired larger scale bed height but low overall column volume by only harbouring a fraction of the 360° piece of an entire radial flow chromatography column to facilitate linear upscaling with minimal investment.
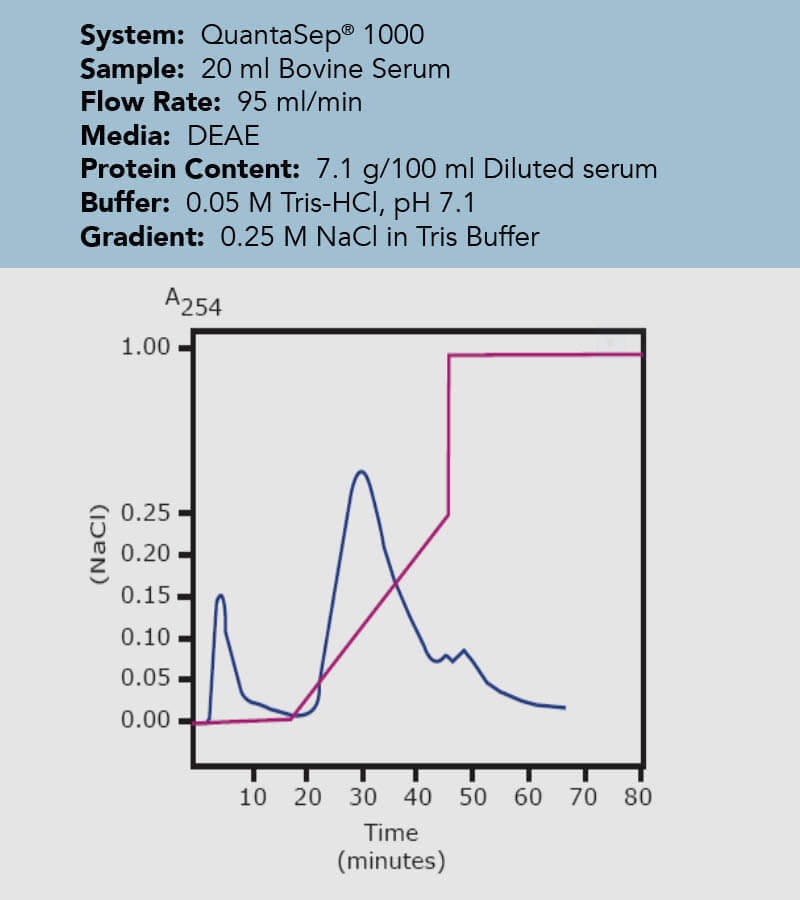
Figure 9: Chromatogram of the initial process developed on a 400 mL Wedge column with the blue line representing the eluent, detected by a UV monitor and the pink line indicating the percentage of buffer B (acidic).
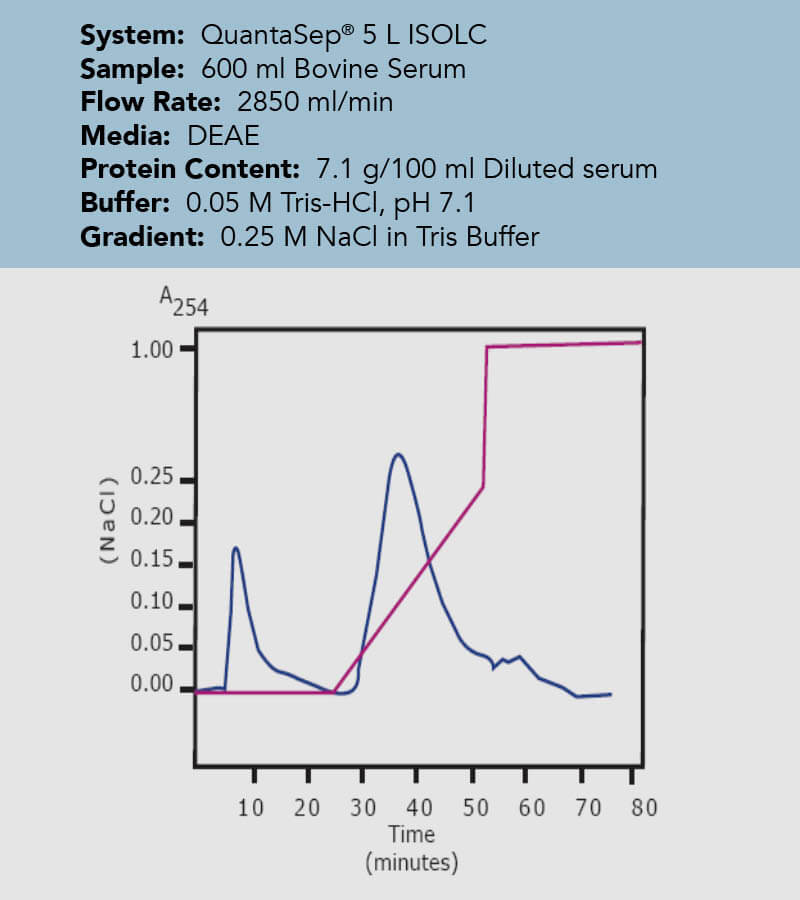
Figure 10: Chromatogram of the final process on a 12 L process radial flow column. The chromatogram is superimposable to the one to Figure 7, process development on the Wedge column with both line colours indicating the same parameters.
How are radial flow columns packed?
With the previously mentioned fixed column volume with redial flow chromatography, making it far more reproducible and reliable as a column design, sufficient packing is not reliant on the skill and experience of the respective technician, but solely comes down to the compressibility of the packing media and its packing end pressure. By accommodating for the resin's compressibility, the defining factor remains as the pressure. This, however, can potentially be automated as to maximize reproducibility and minimize user input to free up their time as well.
Packing with the SuperFlo radial flow column is standardized as to the column being filled through the outlet port with the packing buffer to displace any residual air through the packing ports (on top of the column, making air removal intuitive and uncomplicated), following by flow reversal to actively pump the resin containing slurry into the column through the previously mentioned respective packing ports. The buffer inside the column cylinder is displaced with the resin (for ion exchange chromatography, the SepraFlo resin is recommended) and extruded through (typically) the outer frit of the column due to its larger surface area. This way, there is no uneven compression of the chromatographic bed, now containing the resin.
By actively pumping the slurry into the column, an even column pressure and compression can be reached easily without the need for retroactive adjustments (with a plunger, commonly used on axial flow columns, for example). Pump- assisted packing the column from top enables an active packing to a desired packing end pressure defined only by the chromatographic media and holds quite some flexibility when it comes to pump choice. The flow rate only maximizes /minimizes the column packing speed, not the integrity of the chromatographic bed or the ability to achieve a set pressure threshold. Of course, common packing optimization techniques still have their place when packing a SuperFlo radial flow column, too. As a use case example, packing optimization with a common HIC material, based on highly compressible agarose, yielded the best results when adding salt to pre-'shrink' or compress the media before even packing it and applying the buffer displacement through active packing onto both frits. With these compressible materials, a diluted slurry seems to be desirable, which resulted in an initial concentration of 25% (v/v) for this packing optimization and continuously diluting the slurry with the displaced buffer from the column. This way, pump size, speed and power requirements stay manageable, even with large columns and compressible materials to ensure integrity, reproducibility and ease of use throughout any application, material or column size that might be required.
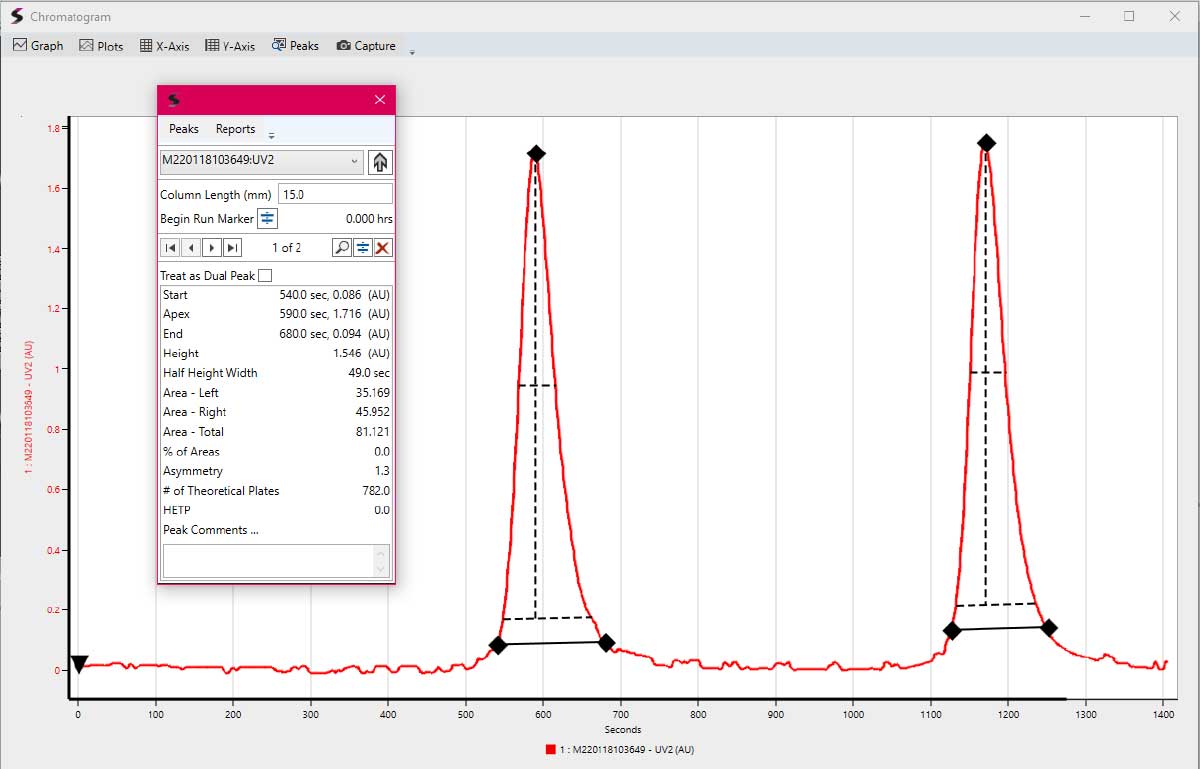
Figure 11: Exemplary chromatogram of a 100 mL acrylic Wedge column with 15 cm bed height to simulate a production packing process. With a dilute slurry and even with low total flow rates, packing highly compressible agarose based affinity materials pose no issues for packing ease, quality and column bed integrity.
Summary
The outlined advantages, reproducible and automizable packing, an more even and more accurate flow distribution, higher flow rates with maintaining crucial linear velocities, lower backpressures and improved separations are culminating in the SuperFlo radial flow column by Sepragen, the original inventors of the radial flow technology. With acrylic and stainless steel materials available in a range from 50 mL to 500 L and more, the column design is limitless as to cater to all requirements in chemical interaction chromatographic applications. Wedge columns are available from as small as 20 mL to easily and effortlessly scale up and explore the separation with the SuperFlo radial flow technology. While there are standard sizes, which are proven to work throughout industries and reliably stay in service over the years, custom options are available for both column size and bed length, as well as materials of the column, the connectors and surface finishing.

Figure 12: Exemplary photographs of a 240 L stainless steel production column (15 cm bed height), a 100 mL acrylic laboratory column (3.5 cm bed height) and a 20 mL acrylic Wedge column (10 cm bed height).