Label-free biomolecular interaction analysis is aimed at understanding the connections among biomolecules and the outcomes arising from these specific associations. This form of analysis plays a pivotal role for medical research and in the pharmaceutical industry. The interactions between biomolecules are of great importance to understand many biological processes. Although certain proteins operate independently, the majority of proteins are only active when they form complexes with other molecules. Biomolecule interactions are fundamental to all cellular processes such as signal transduction and metabolic pathways.
Biolayer interferometry (BLI) studies macromolecular interactions in a label-free setting and is an easy to set up technique in the laboratory. The technique has gained prominence in the field of biotechnology and life sciences. It operates on the principle of measuring changes in interference patterns that occur when biomolecules interact on the surface of a biosensor. BLI offers real-time and dynamic analysis of various biomolecular interactions, such as protein-protein interactions, antibody-antigen binding, and receptor-ligand interactions. This technology provides researchers with valuable insights into the kinetics, affinity, and specificity of biomolecular interactions, enabling a deeper understanding of biological processes. Its simplicity, versatility, and ability to work with small sample volumes make biolayer interferometry an invaluable tool for drug discovery, biomarker identification, and basic research applications.
Key features include:
- Simple label-free detection
- Real-time analysis
- High sensitivity
- Minimal sample consumption and 100% sample recovery
- Handles crude samples – little to no sample preparation needed
- Versatility – technique can be used to measure interactions with various types of biomolecules
- Ease-of-use
- No complicated fluidics
- Easy maintenance of the system
- High sample throughput
- Short assay time
- Reproducible results
- Re-usable sensors
BLI has played a crucial role in medical research and drug development. The versatility of the platform makes it an indispensable tool in multiple scientific development areas, including antibody therapeutics, cell- and gene therapies, and vaccines.
Bringing BLI to the next level with Gator Bio
Gator Bio has introduced a new instrument platform for label-free biomolecular interaction analysis, bringing BLI to the next level. Discover how you can accelerate your biotherapeutics research and drug development with the Gator Bio BLI instruments.

How does Biolayer Interferometry work?
BLI is an optical analytical approach based on the reflection of white light on the surface of a biosensor tip. A shift in the interference pattern occurs when the number of molecules bound to the biosensor tip is changed. The interactions are determined in real-time and analysis of binding specificity, association-dissociation ratios (kinetics) and concentration are measured with high accuracy and reliability. Only bound molecules to the biosensor affect the interference pattern, meaning that non-binding molecules have no effect on the pattern. Consequently, this allows the utilization of crude samples for measurement.
Figure 1 demonstrates how the signal intensity of the reflection beams is translated into a graph, where you can easily monitor the changes in the binding of molecules to the sensor surface.
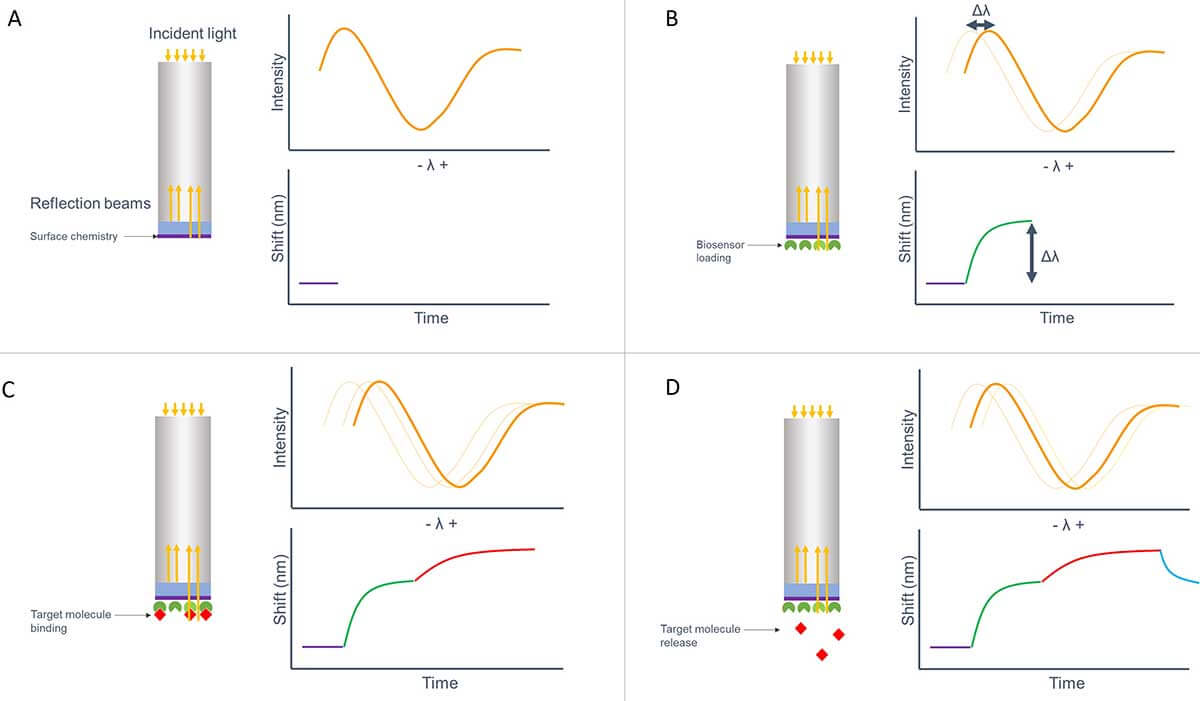
Figure 1. Schematical overview of the different steps of BLI used for a kinetics experiment. Binding is measured real-time, without the need of a label. A. The baseline is set by the reflected light of the surface chemistry. B. The sensor is loaded with the binding ligand. C. The target molecule binds to its binding partner. D. Release of the target molecule.
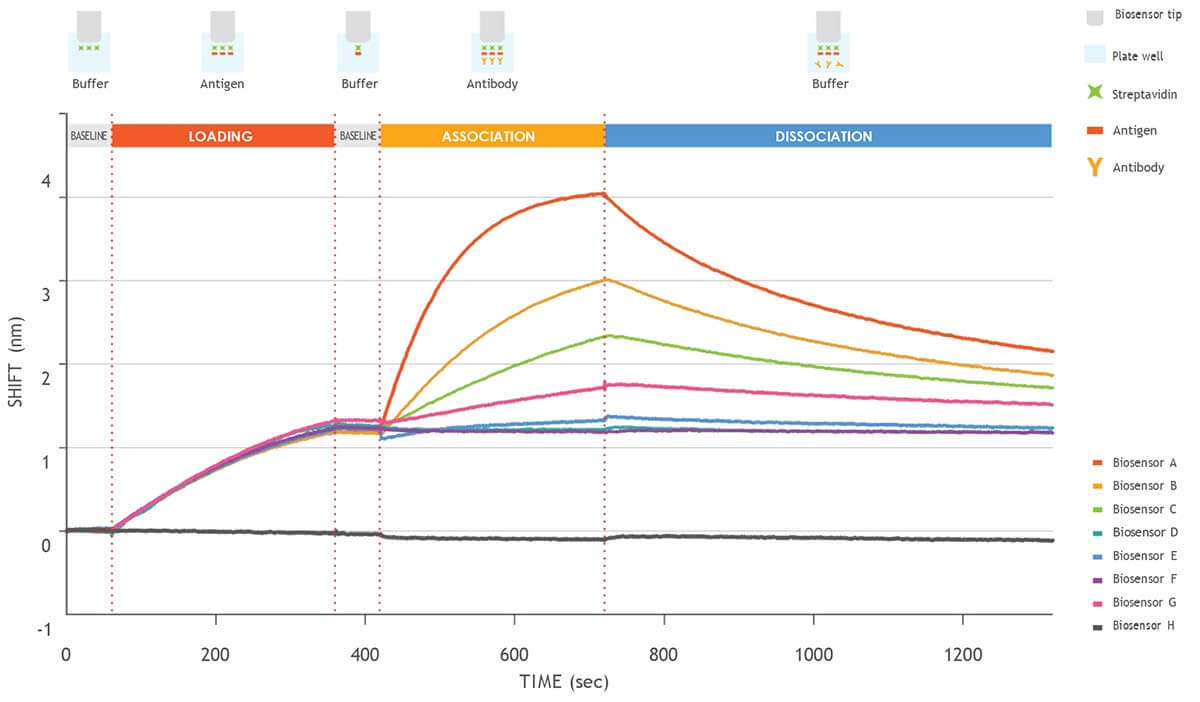
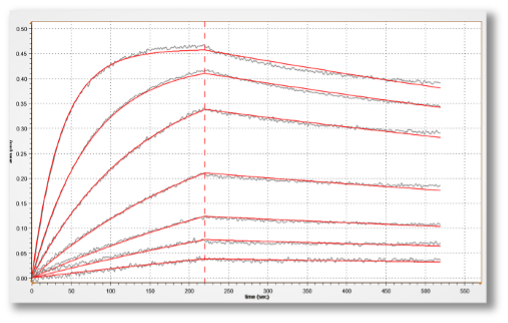
Figure 2. Kinetic analysis of a purified antigen-antibody pair using Gator Bio Streptavidin biosensors.
This graph shows the different steps involved in a standard kinetics experiment (loading, association, dissociation).
Main applications and target molecules for BLI
BLI is a versatile technology that can be used for a range of applications and a wide variety of biomolecules. The most common target molecules researched using BLI are: antibodies, proteins, peptides, small molecules, viral vectors, DNA and lipid nanoparticles.
Quantitation
Measure the concentration of biomolecules such as proteins and antibodies in crude cell culture samples, production harvests, crude lysates or purified samples.
The high-throughput capability and short assay time of BLI makes it very useful for antibody production and development, for example with antibody quantification in supernatants or clone selection.
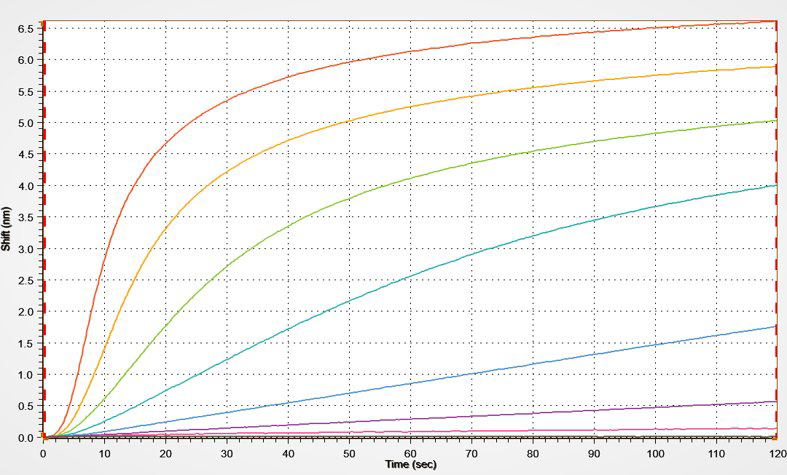
Kinetics
BLI is a very powerful and easy-to-use technique to measure binding kinetics such as association rate and dissociation rate between a biomolecule and its binding partner (on- and off-rates). In addition, affinity strength (dissociation constant) can be determined.
Application areas are:
- Antibody-antigen interactions (i.e. antibody-protein/bacteria/VLP or viral vector)
- Antibody characterization
- Protein-protein interactions
- DNA binding
- Small molecule interactions
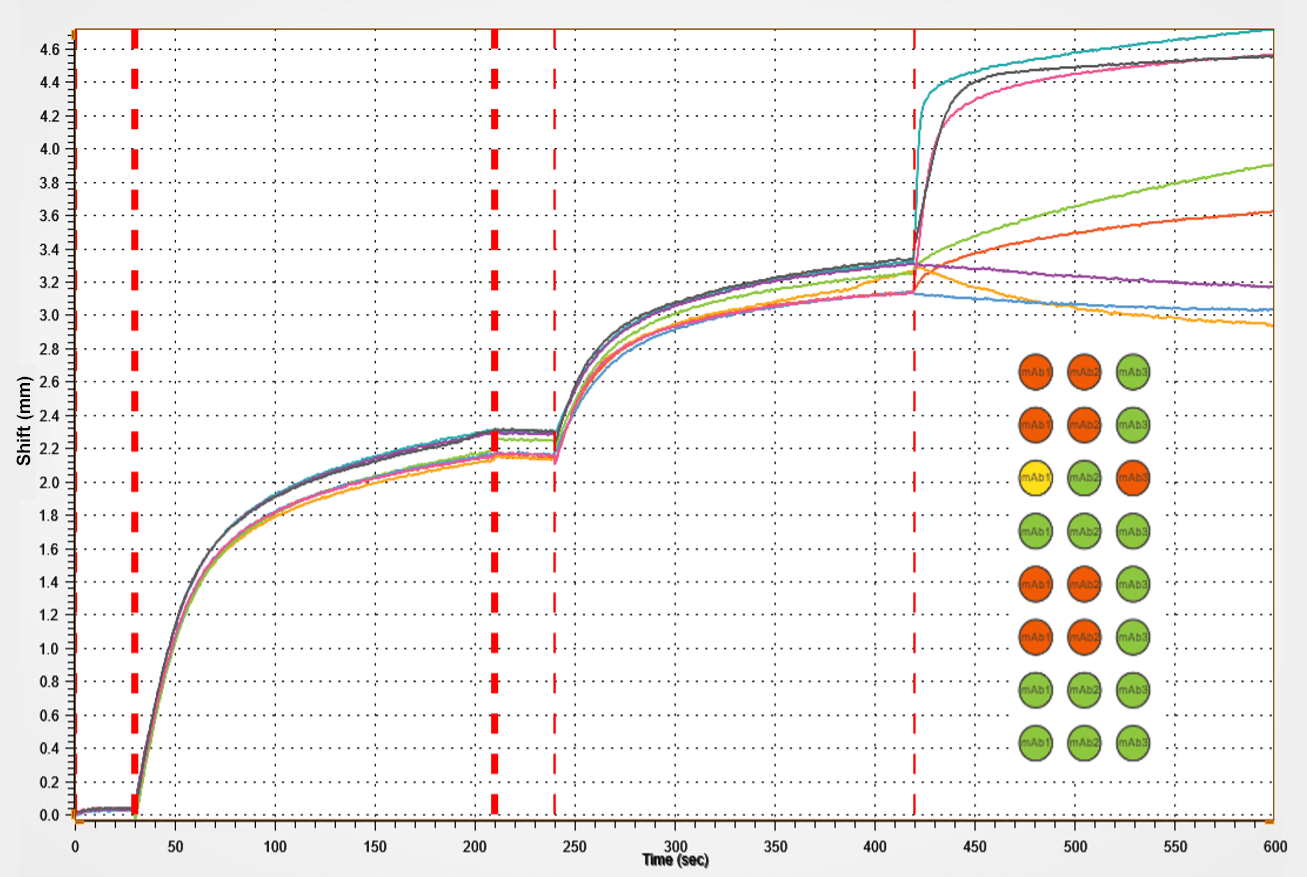
Epitope binning
In antibody drug development, knowing the epitope of an antibody is very important to understand the therapeutic potential. Running epitope binning analysis early on in the drug development pipeline can increase efficiency and save time in the overall drug development process.
In addition, epitope binning can be used to find matching antibody pairs for diagnostic test development and is also used to understand vaccine functionality during vaccine research.